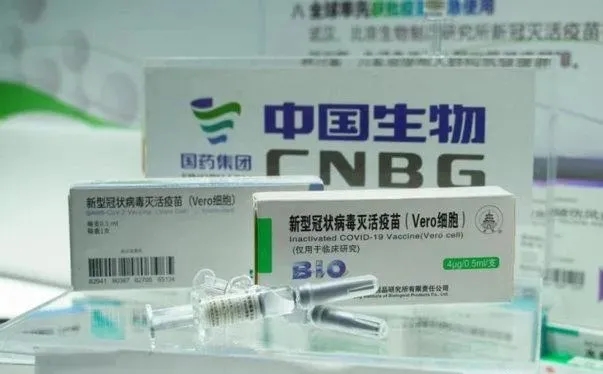
What is the current progress of the recombinant new crown vaccine developed by the Chinese Academy of Military Sciences?
What are the characteristics of this vaccine?
After the successful phase III clinical trial, how long will it take to go public on a large scale?
Who is suitable for vaccination?
Does the vaccine have a preventive effect on the mutated new coronavirus?
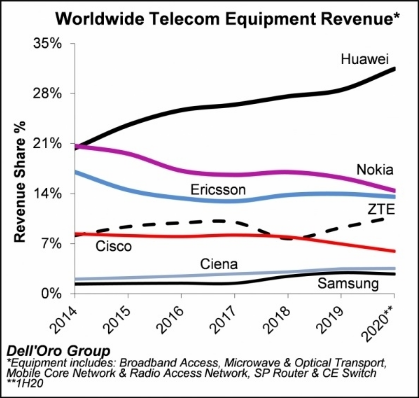
What is the current position of my country's vaccine research and development in the world?

In response to these issues, a reporter from Xinhua News Agency recently interviewed Chen Wei, the winner of the national honorary title of "People's Hero", an academician of the Chinese Academy of Engineering, and a researcher at the Academy of Military Medicine of the Academy of Military Sciences.
"What are the characteristics of the recombinant new crown vaccine developed by the Academy of Military Sciences?"
Chen Wei: We have independent intellectual property rights for this vaccine, which means that we do not need to look at the faces of others to develop our vaccine at any time and on any occasion. When the subsequent vaccine is put into production and application, we can also let the Chinese people get the vaccination as soon as they need it at a lower price.
This is a technologically advanced virus vector vaccine. Its outstanding feature is that it can have both humoral immunity (antibody and neutralizing antibody) and cellular immunity. Since the virus is a parasite, it cannot grow or reproduce by itself. It needs to reproduce in human cells. Therefore, cellular immunity is essential for virus prevention and control. We launched the world's first phase I clinical trial on March 16, and published the phase I clinical trial data on the "Lancet" on May 22. All 108 people vaccinated developed antibodies. The editor-in-chief of "The Lancet" commented on this as "The vaccine is safe and well tolerated. It is the world's first clinical data. A single injection can quickly trigger immunity, which is an important milestone." In this process, we Testing methods and testing indicators have been announced to the world, so that scientific research colleagues in other countries will avoid some detours and speed up vaccine research.
On July 20th, we announced Phase II clinical data for the first time to the world. Phase I and Phase II clinical trials have proved the effectiveness and safety of the vaccine. In June, our vaccine has been vaccinated in specific populations.
Phase III clinical trials are currently being effectively advanced. As the domestic epidemic has been effectively controlled, we need to go abroad to advance Phase III clinical trials and conduct larger-scale vaccine effectiveness and safety evaluations.
"After the Phase III clinical trial is successful, how long will it take to go public on a large scale?"
Chen Wei: Generally speaking, the development of a vaccine has to go through phase three clinical trials, and the test results meet the relevant requirements before preparing for mass production. However, our recombinant COVID-19 vaccine has been prepared for large-scale mass production since the Phase I clinical trial. From the current point of view, the goal of annual output of 300 million is achievable, and we are working hard to expand production capacity. After the Phase III test results come out, our production capacity will also keep up, and we will be ready to vaccinate the people on a large scale at any time and strive for seamless integration.
"Which people do you think are suitable for priority vaccination?"
Chen Wei: First of all, people who are in direct contact with the new coronavirus or have the possibility of close contact, such as those closely related to epidemic prevention, especially front-line medical staff, virus-related researchers, and front-line customs staff. In addition, people with underlying diseases are also our focus. There are also people who are willing to vaccinate themselves.
Regarding the vaccination group and vaccination time, we only put forward opinions and suggestions, and the relevant departments make overall decisions. We mainly prepare high-quality vaccines and can use them in time when the country needs them. This is our responsibility.
"How long will the recombinant new crown vaccine provide effective protection after vaccination?"
Chen Wei: It has only been more than half a year since the new coronavirus was isolated. How long is the validity period of the vaccine? There will not be too many data in the world. It must be data within one year. Our vaccine entered the world's first phase I clinical trial in March, and we only have data within half a year. From the current point of view, the injection in March is still effective. How long can its protection last? We are still advancing related research. At present, we can only speculate based on similar vaccines in the past. For example, the Ebola vaccine will decrease its immune response after the first injection for six months. The second needle is enhanced and can be effective for two years. This is data that can be used as a reference.
"If the new coronavirus mutates, will the vaccine fail?"
Chen Wei: We are a genetically engineered vaccine. We just find the most useful gene and make it into a vaccine. Judging from the current data analysis, the probability of a change in the gene we selected is very low. Up to now, our recombinant new coronavirus vaccine can completely cover the mutated new coronavirus.
In addition, because we are genetically engineered vaccines, once mutations occur and the protective effect is affected, we can use the current vaccines as the basic immunity, and quickly build a more targeted vaccine to boost it, just like giving Software upgrades and patches are the same. This is also the reason why so many countries in the world are making genetic engineering vaccines-it is a new generation of technology and a sunrise technology that we need to vigorously develop in the future.
"What do you think of our country's new crown vaccine development process in the world?"
Chen Wei: There is no doubt that our country is in the first phalanx of the world's new crown vaccine research and development. We currently account for more than half of the vaccines announced by the WHO that have entered phase III clinical trials. This data is already very telling.
 小任班长的博客
小任班长的博客